Implantable Medical ID Approved By FDA
By Rob Stein
Washington Post Staff Writer
Thursday, October 14, 2004; Page A01
A microchip that can be implanted under the skin to give doctors
instant access to a patient's records yesterday won government
approval, a step that could transform medical care but is raising
alarm among privacy advocates.
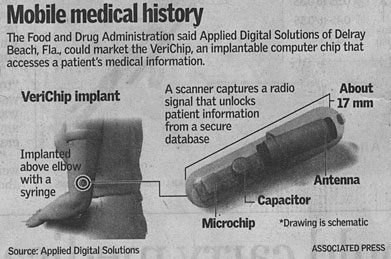
The tiny electronic capsule, the first such device to receive
Food and Drug Administration approval, transmits a unique code
to a scanner that allows doctors to confirm a patient's identity
and obtain detailed medical information from an accompanying
database.
Applied Digital Solutions Inc. of Delray Beach, Fla., plans to
market the VeriChip systems -- the chips, scanners and computerized
database -- to hospitals, doctors and patients as a way to improve
care and avoid errors by ensuring that doctors know whom they
are treating and the patient's personal health details.
Doctors would scan patients like cans of soup at a grocery store.
Instead of the price, the patient's medical record would pop
up on a computer screen. Emergency room doctors could scan unconscious
car accident victims to check their blood type and medications
and make sure they have no drug allergies. Surgeons could scan
patients in the operating room to guard against cutting into
the wrong person. Chips could be implanted in Alzheimer's patients
in case they get lost.
"In hospitals today, many deaths occur because people aren't
able to communicate timely enough their medical information or
because of wrong information," said Scott Silverman, the
company's chief executive. "With VeriChip, you'll be able
to have accurate information even if a patient can't talk. It's
a way to modernize our antiquated system of medical records."
The approval was immediately denounced by privacy advocates,
who fear it could endanger patient privacy and mark a dangerous
step toward a Big Brother future in which people will be tracked
by the implants or required to have them inserted for surveillance,
identification and other purposes.
"Once the technology is out there and is available, it raises
the very real possibility that people in a position to require
or demand it will begin to do that," said Katherine Albrecht,
who has campaigned against such devices. "It would obviously
be possible to inject one of these into everyone. In the post-9/11
world, we are already racing down the path to total surveillance.
The only thing missing to clinch the deal has been the technology.
This may fill that gap."
The VeriChip technology was developed to track livestock and
has been implanted in about 1 million cats and dogs to identify
lost or stolen house pets. But the technology has a variety of
other potential uses, and the company has already sold about
7,000 chips for human use, about 1,000 of which have been implanted.
Mexico's attorney general announced in July that he had one of
the devices injected into his arm, as had about 160 of his lieutenants,
to control access to high-security offices. In bars in Amsterdam
and Barcelona, patrons can have the chips implanted to allow
them to enter exclusive areas and keep track of their tabs.
The company is investigating other applications, including using
the chips as "electronic dog tags" for soldiers, creating
"smart guns" with built-in scanners that ensure they
can be fired only by someone with a corresponding implant, and
enabling stores to verify a customer's identity before accepting
a credit card.
"That same scanner in a Wal-Mart that is used to bar code
your goods can be used to identify you when you present your
credit card to make sure someone hasn't stolen it and your identity,"
Silverman said.
Spurred by South Americans seeking ways to trace kidnap victims,
the company has also developed a device that allows satellites
to pinpoint a chip's location, but it has no immediate plans
to market that gadget.
The company hopes the FDA approval, however, will speed the proliferation
of the chips for medical and other uses.
"We believe that this application is going to drive acceptance
of the product," said Angela Fulcher, vice president for
marketing and communications. "If you have a chronic disease,
where getting information to health care providers quickly may
mean life or death, that population is going to be more accepting
of this technology."
The company hopes to kick-start use of VeriChips by donating
about 200 of the $650 scanners to trauma centers. The chips,
which are the size of a grain of rice, will cost about $200 apiece.
The devices are injected with a syringe under the skin of the
upper arm in a quick, painless procedure.
The accompanying scanners and software ensure that the personal
information unlocked by the 16-digit code is only available to
those designated by the patient, Silverman said.
"Even if people access your unique identification number,
which would be extremely difficult to do, it doesn't give them
access to your database. We're confident in the security measures
we've taken," Silverman said.
Opponents argue that the medical benefits are marginal at best.
Patients can already wear bracelets that alert doctors to their
identities and special medical needs, and few medical errors
are actually caused by patients being misidentified, they say.
But the potential for abuse is great, they caution.
"Over the long haul, any place where there's a surveillance
camera today, five or 10 years from now will have these . . .
readers. You'll walk into a 7-Eleven, and they'll take your picture
and scan your number," said Richard M. Smith, an Internet
security and privacy consultant in Boston. "If we start
carrying these tags it makes a perfect way, either by private
security companies or the government, to keep track of us."
Marc Rotenberg, executive director of the Electronic Privacy
Information Center in Washington, said he was concerned that
people might be forced to get the implants.
"When you put an identification tag under a person's skin,
you make it impossible for a person to remove the tag, much like
branding cattle," Rotenberg said. "The most likely
applications would involve prisoners and parolees, and perhaps,
one day, persons in the United States who are not citizens. I
think there needs to be some legislation put in place to prevent
abuse."
Silverman dismissed the concerns, saying abuse would be technologically
difficult and the benefits would far outweigh any theoretical
risks.
Home